Digital solutions for management of trainings including qualification matrix
Digital Life Sciences GmbH offers GxP-compliant documentation solutions, which primarily address document-based processes of manufacturing / production and quality management. From document control and deviation management (DC), CAPA, digital personnel file and training management including qualification matrix to change management (CC), batch documentation and contract management, we offer our customers individual solutions according to their wishes and needs.
We develop and market software for the end-to-end digitization of business processes and industry-specific specialized procedures.
Our focus is on the control of records and archiving, the control of documents as well as production-related QM processes. Our products have already been introduced and successfully validated at numerous companies. Our digitization solutions have also been successfully audited by TÜV in accordance with the guidelines of the ISO standards 9001 and 13485 as well as during inspections of the regional council (Germany). We are pleased to have a customer base of over 130 customers. The majority of our customers are projects from the life sciences sector that require validation.
Learn more about our software solution for your training management.
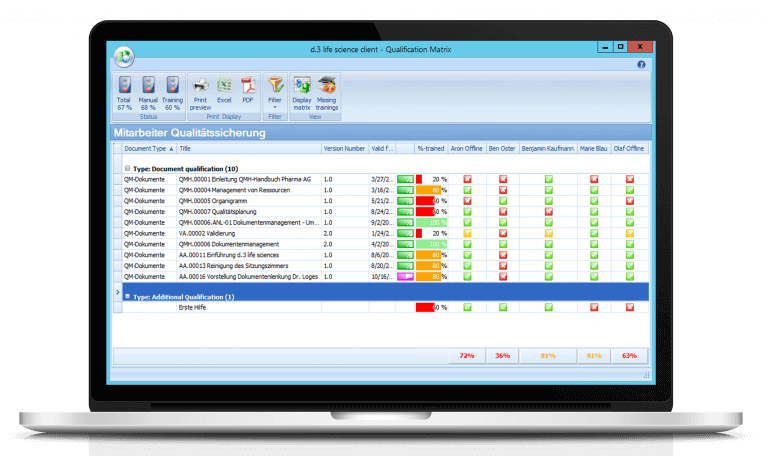
Training management — digital planning & documentation of the qualification level of your employees
Do you still keep manual EXCEL lists to map the qualification matrix? That doesn’t have to be. With our training management solution you can digitally plan and document the qualification level of your employees. Document-based trainings are supported as well as external trainings or further education. The consistent integration of the training module into the “Controlled Documents” module also completes your digitization strategy.
Our solution is designed to support in the sense that only employees who have been successfully trained are authorized to carry out the corresponding activities. In addition, an analysis of the training status based on a document, an employee or a qualification profile is conveniently possible. Plan your training measures digitally.
Whenused in combination with our “Controlled Documents” module, the release of a new document revision automatically generates the associated training tasks.
Our digital training management solution provides support for document trainings, face-to-face trainings and external trainings. Furthermore, an optional learning success control based on multiple choice tests is possible. Configurable repeat intervals, automatic generation of training sheets and participant lists as well as automatic delivery of relevant documents when changing departments can also be set.
Maintain control and overview of employee training, requirements, and qualifications of your employees. All this is automated and digitally documented by our training management software solution.
Regulatory requirements:
- ISO 9001:2015, chapter 7
- ISO 13485:2016, chapter 6
- EU GMP Guideline Part 1, chapter 2
- EU GMP Guideline Part 2, chapter 3
- Ordinance for the Manufacture of Medicinal Products and active Pharmaceutical Ingredients section 2, §4
- FDA 21 CFR 211 Subpart B
Comprehensive solutions for all aspects of electronic documentation
With document control, training management, deviation management (DC), change management (CC) and corrective and preventive action (CAPA) as well as contract management, we offer you comprehensive digitization solutions according to your individual wishes and needs. Our solutions are specialized on the life cycle of its specifications and form sheets.
Our basic product d.3 is meanwhile used by more than 12,500 customers worldwide as a central ECM/DMS/archive system. Our solutions are a complementary supplement to d.3 and are primarily designed for document-based processes of manufacture / production and quality management.
Our company building is located on the campus of d.velop AG in Gescher — we will gladly be at your disposal for a detailed consultation. You can reach us by phone under +49 (0) 2542 — 20201 0 or via our contact form.
We are looking forward to your inquiry.
Are you looking for a solution for your training measures?
Then take a look at our solution now