From individual documents to our document management system and process oriented dossier management, our module “dls | technical documentation” supports and simplifies the creation and updating of your technical documentation. For example in the context of the requirements of the Medical Device Regulation (MDR). Our process-oriented dossier management is versatile. For example it includes the creation of site master files, tool logbooks, drug master files, QM documentation, project folders, validation documentation and much more.
How do you benefit from the Technical Documentation software?
Make manual documentation of dossiers a thing of the past — instead, manage them completely digitally.
Let untidy file servers with multiple storage locations and redundant folder structures be a thing of the past.
Protect your data from unwanted actions of unauthorized users as well as from destructive forces and stop worrying about imminent data loss.
Let the modules be configured according to your wishes and preferences to facilitate the onboarding training for your colleagues.
Digitally create and update your technical documentation within the requirements of the Medical Device Regulation.
Minimize process runtimes for creating, revising and approving your dossiers and reduce unnecessary time spent on searching and compiling the latest documents.
Use d.velop documents (formerly d.3ecm) as your central information platform on which you can retrieve all your data at short notice.
Access your data at any time. It does not matter where you are.
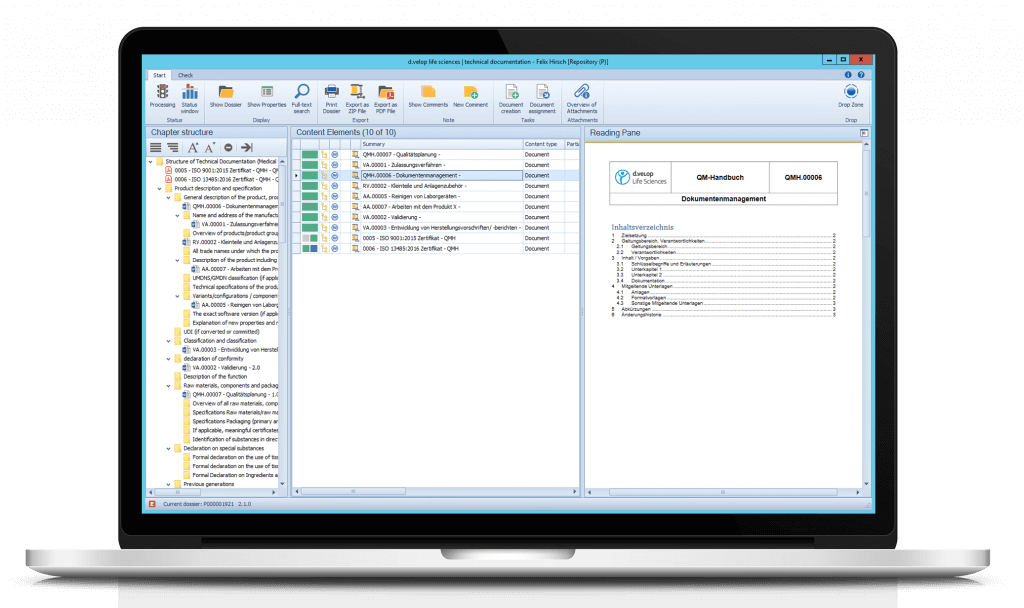
Here are some features of the Technical Documentation

Moreover, you can directly adopt documents from the eDMS/eQMS using the dossier template. Add additional documents and attachments to the dossier application and assign them to the respective chapters via drag & drop.
Print your dossiers or export them as a ZIP file or PDF/A. It is also possible to print and/or export only parts or certain chapters of the dossier.
Using the Drop Zone, non-controlled and controlled documents can be stored via drag & drop. Documents can also be assigned directly to the respective nodes/chapters in the dossier via drag & drop.
For each document, a preview can be displayed within the flexibly customizable reading pane.
Create templates and chapter structures for your dossiers according to your needs and define responsibilities for each part. Dossier templates, e.g. for the MDR tree structure, are automatically included when the module is implemented.
Would you like a live insight into the software?
Get a live insight into the possibilities of implementing a process-oriented dossier management by means of a sample use case in only 45 minutes. Learn how our solution can support and simplify the creation and updating of your technical documentation within the requirements of the Medical Device Regulation (MDR).
Here are some features of the Technical Documentation
- Manage templates for different structures of your dossiers
- Manage tasks for the editors of individual chapters of a dossier (workflow)
- Seamlessly integrate into our Document Control module
- Check the completeness of your dossiers
- Check the current editing status of your dossier
- Create versions of your dossiers and access old versions easily
- Create comments with different priorities
- Export the entire dossier or selected parts as a PDF or ZIP file
- Print the entire dossier or selected parts of it
- Use the integrated electronic and GxP-compliant signature as well as audit trail functions
- Classification of dossiers via attributes in ECM d.velop documents (formerly d.3ecm)
Regulatory requirements for Technical Documentation
- ISO 13485 MPG
- Medical Device Regulation (MDR) — EU Directive MDR 2017/745
33 Good reasons for a cooperation with Digital LS
You’re not convinced yet? Find out about 33 good reasons speaking for a cooperation with Digital Life Sciences GmbH. We will show you reasons from the provider’s point of view, from the software point of view and other general reasons that distinguish us.
Customer reviews of Technical Documentation
“We have been working together with d.velop AG very successfully and contentedly for years. Looking for a reliable partner for the technical documentation of our medical products, we stumbled upon the life science client. With the transition to the new structure of EU MDR, we were able to fully exploit the potential of the Life Sciences Client for us and thus facilitate the work of document creation and improve acceptance in the company.”

You might also like…
The Technical Documentation is a component of the d.velop life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other types of documents — you can create, revise and sign them all digitally with the document control software.

Training Management software
Extend the “Document Control” module to actively plan and record the qualifications of your employees with our training management software.
QM process (Complaint | DC | CAPA | CC)
Digitalize your ISO processes. Control your production-related QM processes using digital workflows.
Frequently asked questions (FAQs) about Technical Documentation
Are there templates for the structure of the dossier?
Templates are available for frequently used documentation, e.g. according to Annex II of the MDR 2017/745. On the basis of a structure used by you, you can also amend templates yourself or have them created as part of the introduction of the module.
Can chapter structures be adapted?
You can customize chapter structures and dossier templates or create your own.
How do I ensure that an employee adds all relevant documents to the dossier?
Using the tasks ‘Placeholders’ and ‘Document creation’ you specify which documents the dossier must contain before it can be released.
Can I use documents from the file system in the dossier?
Documents from your desktop or from the file directory can be used in the dossier. They are stored in the document management system and used from there in one or more dossiers.
Can several employees work on one dossier?
For each dossier, a main person responsible is identified, who also receives the reminders for completion or periodic review. Individual chapters of the structure can be transferred to a person partially responsible for processing.
Can documents be used more than once?
Documents are added once to the contents list of the dossier and can then be inserted at several positions in the structure. Documents can also be used in several dossiers.
Why does one use the technical documentation for the approval of a medical device?
Technical documentation is used for the approval of a medical device to provide regulatory authorities and others involved with information about how the device works, who will use it, and how it can be used safely.
Are the documents in the dossier updated when new versions are released?
When a dossier is released, the version is ‘frozen’, changes are only possible in a subsequent version. With the overview ‘Version statuses’ you get an overview of the document versions. To adopt new versions, create a new version of the dossier and decide which version of the documents to adopt.
For documents added to the dossier as attachments, the valid release version is always used.
Can individual documents from the dossier be printed?
Printing of documents is possible, the function depends on the specifications from the document management system (controlled — not controlled).
Is a partial export of the dossier possible?
Export as ZIP archive or PDF file is possible for the whole dossier or for selected chapters / documents.
Is the data exported from the dossier protected?
For the ZIP file, the protection is preset with a password. Furthermore, you can preset the creation of PDF/A files to prevent printing and copying and enter a watermark for each export.
Is the export logged?
The actions are recorded in the audit trail of the dossier, including the output as a ZIP or PDF file and the export of individual documents.
The audit trail stores the date and time of an activity for the actions listed below. Moreover, the ID of the user triggering the activity is stored in the audit trail entry.
Which file formats can be used in the dossier?
Documents can be inserted into the dossier regardless of the file format (PDF, MS Word, Excel, PowerPoint, PNG, JPG, TIFF, Visio)
How can our software help you with technical documentation?
Our technical documentation software is an application primarily used for managing technical documentation (“dossiers”). It can manage a large number of dossiers in the process. The software allows you, for example, to create technical documentation in accordance with MDR (EU Medical Devices Regulation). The goal here is the audit-proof management of all documents and content that arise in the life of a medical device.
We will be happy to show you how you as a user can benefit from our software for technical documentation in your company in the future in a live WebCast on the software.
Overview Medical Device Regulation (MDR)
Medical Device Regulation (MDR) — Regulation 2017/745 (EU) on medical devices
The German equivalent for the term Medical Device Regulation (MDR) is “Medizinprodukteverordnung”. The European Union (EU) Regulation 2017/745 will become binding throughout Europe on May 26, 2021. In this article you will find information on content and implementation regulations for the medical technology industry.
EU regulation tailored to medical technology
Already on April 5, 2017, the “Regulation (EU) 2017/745 of the European Parliament and of the Council on medical devices” became effective. It replaces the previously valid “Directive 93/42/EEC on medical devices”. This actually means for the medical technology sector that the EU regulation 2017/745 substitutes for the following previous guidelines:
- Directive 93/42/EEC concerning medical devices (MDD);
- Directive 90/385/EEC, active implantable medical devices — Active Implantable Medical Devices (AIMDD)
The EU bodies opted for a separate regulation with regard to Directive 98/79/EC on in vitro diagnostic medical devices (IVD). This guideline did not find its way into the medical device regulation. It will be replaced by the separately worded EU regulation “In-Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR).
Binding throughout Europe as of May 26, 2021
In contrast to the superseded directive, the EU Parliament’s new regulation has gained an international profile. Its binding validity extends to all EU member states from the key date of May 26, 2021. It should be noted here that as of October 2019, no national legal acts have yet been adopted by the individual member states. This also applies to German specifics, requirements and penal provisions to be enacted. A corresponding draft for a German implementing law does exist. Nevertheless, the Federal Ministry of Health anticipates transition periods.
According to the present text of the law, a transition period of three years (05/26/17 — 05/25/21*) applies during implementation. After the deadline at the latest, you as a manufacturer are obliged to submit an MDR certificate. This is the only way you are allowed to introduce a product to the market for the first time.
*In the wake of the COVID 19 pandemic, the effective date of the regulation was delayed to May 26, 2021
Aim and necessity of the new EU regulation
In essence, the new regulation aims at an optimized, uniform regulation for the market launch of medical technology products. The focus is on the factors of product safety and product quality.
For you as a market participant and user, the new EU regulation consists of a mix of familiar content from the previous Directive 93/42/EEC and far-reaching changes. Among the essential, substantive and procedural content for you and your industry are the following contents of the Brussels Regulation:
Rules for the classification of medical devices
New terms and rules lead to changes in the assignment of existing products to partially modified product classes. For example, the product range of “stand-alone” software is affected by these changes.
Conformity evaluation procedure
Following the set of rules for classification, the conformity assessment procedure now also varies depending on the class.
Technical Documentation/Document Management System
The technical documentation according to Medical Device Regulation proves to be much more detailed. Benefits of control and documentation for the companies become clear when looking at the new underlying tabular scheme in detail. The subdivision into individual categories in a direct link with the associated detailed requirements forms the basis for a detailed technical documentation.
Clinical evaluations/clinical trials
Significantly increased requirements characterize this action item. This applies in particular to high-risk product groups.
Company responsibility/safety officer
A significant change for your company results from the requirement of the EU bodies for a responsible person in their organization to be accountable for regulatory compliance.
Quality management
The FDA Medical Device 21 CFR 820 (FDA = U.S. Food and Drug Administration) stipulates the requirements for management systems of medical device manufacturers. In this function, Device 21 CFR 820 is the counterpart to ISO 13485. The core requirement is that procedural instructions such as document control, procurement, development and production are documented and implemented analogously.
Market surveillance mechanisms
In particular, the mandatory use of the EUDAMED database provides greater transparency. It is available to both the public and direct competitors as an information medium. The characteristics of each medical device are mapped by means of appropriate links. Supply chain traceability is also possible.
Ways to comply with the Medical Device Regulation
To fascilitate timely compliance with the new regulation, you are called upon to act, if you hold a responsible position in your company. A coherent plan for the transition of your company to the Medical Device Regulation, both in terms of time and content, is a prerequisite for your success. High priority should be given to the life cycles of your medical device portfolio. Existing MDD certifications will lose their validity on May 26, 2024, according to EU regulations. Please note in your planning that your products already certified according to MDD must also complete the new conformity process to be established according to MDR. A “grandfathering” is not guaranteed.
In the transition phase, you are particularly challenged in your function as a manufacturer or provider. The highest priority is to designate and train the person responsible for regulatory compliance in your facility (MDR Article 15). Updates to internal quality assurance procedures, documentation, and modified product classification are mandatory.
Against this background, the following standardized procedure according to the following 4 points is recommended:
- Implementation of the conformity evaluation related to the product
- Completing a declaration of conformity
- “CE” marking of products
- Registration of company and products in the EUDAMED database.
Conclusion and criticism of the new regulation
With the MDR, EU officials have set out to create a contemporary, uniform solution for quality management and market release of medical devices. Great attention is paid to the quality and safety of products. Perhaps you also belong to the group of small and medium-sized companies in the medical technology sector that fear over-regulation by the extensive set of regulations and additional auditing bodies?
In particular, the evaluation of compliance by the “compliance manager” to be recruited is likely to become a personnel problem during the start-up phase. In a case of hardship, a shortage of certified auditors could delay the certification needed for market release. Medical technology industry associations express fears that a bottleneck in certifications could have a negative impact on the market launch of innovative and marketable products.
Quick contact
You have a question about Technical Documentation?
Our sales team will help you promptly and gladly.

