Software for your SOP management
Whether work / process instructions (SOPs), process descriptions, test specifications, operating instructions, contracts or any other types of documents — you can create, revise and sign them all electronically with our “Document Control” module. In the process, the document circulation is set through configuration. Different (single or multilevel) document circulations can be stored for each document template. Thus, it can be customized to your needs and procedures. The system automatically assigns corresponding “Periodic Review” tasks to the appropriate employees. The integrated reminder and escalation management system notifies when deadlines are exceeded and tasks are past their due date. The participation in the automatic document circulation is confirmed by the electronic and GxP-compliant signature. Integrated audit trails for every document increase traceability.
How can you benefit from the Document Control software?
Digital management
Digitally manage your specification documents (e.g. work and process instructions, test specifications, hygiene plans), forms, contracts and other document types.
Dispatch at the touch of a button
Digitally handle the document distribution and the signature circulation at the touch of a button and focus on your core business.
Increased data security
Protect your data from unwanted interference by unauthorized users and from destructive forces.
Individual configuration
Let the modules be configured according to your wishes and preferences to facilitate the onboarding training for your colleagues.
Minimization of process runtimes
Save time by minimizing process runtimes for the creation, revision, release and distribution of your specification documents.
Reduction of resources
Reduce unnecessary printouts and paper on your desk. This spares the environment, your nerves and your money.
Central information platform
Use the d.velop documents (formerly d.3ecm) as your central information platform on which you can retrieve all your data on short notice.
Constant availability
Access your data at any time. It does not matter where you are.
Get to know our software better

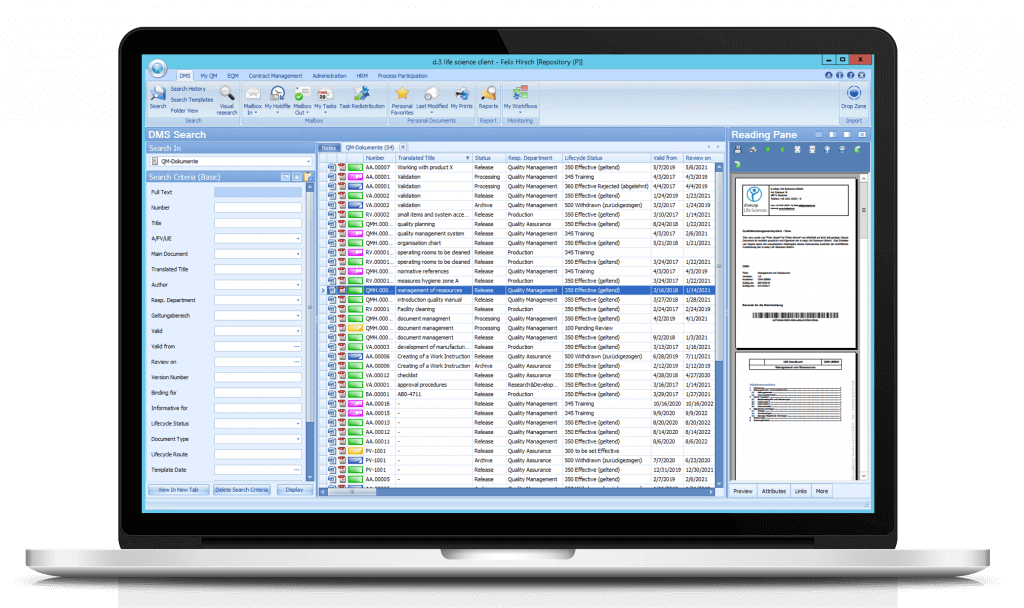
All documents and their properties that match your search criteria within the DMS search are displayed here.
You open the menu by clicking on the dls | eQMS icon in the upper left corner. If you choose a menu item or a submenu item, in the Overview Window the according result list is displayed.
Controlled and non-controlled documents as well as any document types and file formats can be stored by drag & drop via the Drop Zone.
For each document, a preview can be displayed within the flexibly customizable reading pane.
The application window of the dls | eQMS displays the following menu items depending on the user, licensed module and authorization:
- DMS
- My QM
- Process Participation
- Administration
- Training Management
- Contract Management
- HRM
The document status differentiates between:
- Processing (red)
- In circulation (yellow)
- Released (green)
- Withdrawn (blue)
- Training phase (purple)
Documents can be found using various search criteria and attributes. The search result is displayed in a results list on the right side in a tab. Of course, a full-text search as well as a search via visual elements is also possible.
Would you like a live insight into the software?
Get a live insight into the possibilities of the document control in just 45 minutes with a sample use case. Learn how you can electronically create, revise and sign your work/process instructions (SOPs), process descriptions, test specifications, operating instructions, contracts or any other document types by means of the solutions of Digital Life Sciences.
Some features of the Document Control
- Creation of regulated/controlled documents within the system based on released templates
- The templates may also be controlled documents
- Management of specification documents and form sheets
- Digital document signature using integrated GxP-compliant signature — purely software-based
- Full-text search based on document content and document attributes (meta data)
- Individual and dynamic compulsory watermark
- Easy creation of report lists
- Controlled and logged printing
- Comparison of document versions and visualization of differences
- Integrated archiving on the basis of the ECM d.velop documents (formerly d.3ecm)
- Windows and web frontend
- Periodic review
Which regulations must be observed for controlled documents?
- ISO 9001:2015, Chapter 7.5
- ISO 13485:2016, Chapter 4
- FDA 21 CFR Part 11
- EU-GMP guideline, Annex 11
- WHO guidance on good data and record management practices
- EFG-Votum V11002: Requirements regarding the electronic data storage
- EFG-Votum V11003: Requirements regarding electronic signatures and initials
33 Good reasons for a cooperation with Digital LS
You’re not convinced yet? You’re not convinced yet? Find out about 33 good reasons speaking for a cooperation with Digital Life Sciences GmbH. We will show you reasons from the provider’s point of view, from the software point of view and other general reasons that distinguish us.
Customer review on the Document Control
“Years ago, we used to track the status of documents such as work instructions, employee qualification records and batch reports using an Access database or on paper. However, it was tedious to keep the documents up to date and to manage them in an audit-compliant manner. In 2012, we switched to the digital document management of Digital Life Sciences, which was a quantum leap. Today we can create, find, edit and coordinate documents much faster. This saves working time and costs.“

You might also like…
The Document Control is a component of the Digital Life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Training Management software
Extend the “Document Control” module to actively plan and record the qualifications of your employees with our training management software.

E‑learning software
Use the new software “E‑Learning” to train your employees digitally. Create an e‑learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.
QM process (Complaint | DC | CAPA | CC)
Digitalize your ISO processes. Control your production-related QM processes using digital workflows.
Frequently asked questions (FAQs) about Document Control
Can SOPs be divided into different categories?
For the administration of QM documents, the dls | eQMS can manage the three document categories ‘QM manual, process instruction and work instruction’. These three document categories reproduce different hierarchical levels of the QM documentation. The QM manual includes universally valid documents such as policies, for example, and refers to the other two underlying hierarchy types for a detailed definition of operating instructions.
Can the document circulation be configured?
Document circulations (e.g. review, approval, release, training, entry into force) can be flexibly configured and adapted to your company requirements. The circulation is linked to the document template, so a different circulation could be defined for each template.
Can specifications be set regarding the selection of persons for the circulation steps?
You can determine whether and which employees and/or employee profiles can be selected in each individual step. You can also determine whether a minimum or maximum number of people are involved in a step and specify a particular person/profile or make it mandatory.
Are released QM documents automatically put into force?
The entry into force is always the last step in a document circulation and usually takes place automatically at the end of the scheduled training phase. It is however also possible to put the documents into force manually.
Can the circulation of documents be supplemented by a periodic review?
In a document circulation you can decide if you want to have a periodic review task sent. If a periodic review is planned, the releaser receives a date proposal based on the configuration (e.g. 2 years after the entry into force) and a periodic review task is sent appropriately in advance.
The periodic review task can be sent to different persons or activity profiles (e.g. reviewer, approver, releaser).
How do employees receive QM documents?
Independently of the additional functions in the ‘Training Management’, you can define a document distribution list on the basis of which the ‘Binding for list’ is created for a document. If document distribution is used, a first distribution list is specified in the document circulation. In the first distribution list you select those users who will receive the task for the read-and-understood training after the document is released. In connection with the processing of this task, a sub-distribution option is available. The employee can distribute the document to other employees in connection with the read-and-understood training.
Can a document be edited by several employees?
A document is assigned to an author who is reserved for editing. However, the authorship can be transferred to another employee so that he or she can add further content if necessary.
Can a numbering scheme for SOPs be configured?
By means of a numbering scheme a unique number can be assigned to each document in the dls | eQMS. Hierarchical numbering is also available here, which has an effect on the release processes. For example, an attachment created with a hierarchical numbering system can only be released if the superordinate document is released.
How are further applicable documents and attachments managed?
One or more further applicable documents and/or attachments can be assigned to a controlled document. They are listed in the document properties and in the preview and can also be accessed directly here.
You can configure that the author of a document receives a notification when a further applicable document or attachment is released in a new version.
Are printouts of a controlled document documented?
With appropriate authorization, an employee can print controlled documents. During controlled printing, he or she selects the extent to which the document should be printed (additional pages with audit trail information, electronic signatures etc.). A printing reason must be specified and the printout is logged in the properties.
If the printout is reported as returned/destroyed, this is noted in the system using the function ‘Confirmation of Printout Return’.
How is the signature implemented within the document circulation?
An electronic signature is implemented for all actions requiring signatures (document circulation, read-and-understood tasks etc.). It meets the requirements of the FDA and the GMP guidelines and replaces the personal signature on a printed document. This function enables responsible persons to provide the necessary signatures even at a distance. Each signature is recorded in the audit trail and printed on an additional page with the document.
Are all versions of a controlled document managed?
Controlled documents are managed in a minor and major versioning. There may be multiple editing versions if the review/approval/release has been rejected in the document circulation. The author creates a new editing version and restarts the circulation. All versions of a controlled document can be viewed by authorized users. A direct document comparison of two versions is also available here.
Quick contact
You have a question about document control?
Our sales team will help you promptly and gladly.