Software for your complaint management
Control your production-related QM processes such as complaints with our digital workflows. Our integrated solutions manage your previously paper-bound process from capture to completion completely digitally. The workflow system automatically forwards the digital form to the next instance; if deadlines are exceeded, the reminder and escalation management takes effect. Of course, an absence management is also included for the task routing.
What benefits does the Complaint software offer you?
Consideration of specifications
Changes to products, processes or systems are carried out in accordance with regulatory and internal specifications.
Transparency
Every authorized employee sees the current status of all complaint processes – even if he or she is not involved in the process.
Linking to other processes
From complaints, corresponding Deviation Controls (DCs), Corrective And Preventive Actions (CAPAs) and Change Controls (CCs) can also be generated.
Individual configuration
Let the modules be configured according to your wishes and preferences to facilitate the onboarding training for your colleagues.
Central information platform
Use d.velop documents (formerly d.3ecm) as your central information platform on which you can retrieve all your data at short notice.
Reduction of process costs
The costs of administration as well as the runtimes of the processes are reduced, the quality of the results is increased.
Process safety
After each step, the workflow system ensures that the complaint is forwarded to the person in charge. The escalation system takes effect in case of missed deadlines. The escalation system takes effect in case of missed deadlines.
Increased data security
Protect your data from unwanted interference by unauthorized users and from destructive forces.
Constant availability
Access your data at any time. It does not matter where you are.
Reports
Create reports and statistics about your complaints.
Get to know our software better

The digital forms are also converted to a PDF/A in parallel. This way, employees who are not involved in the process can also gain insight into the workflow and the document can be shared with external parties if required.
What is the Complaint Process?
The CP (Complaint Process) allows drug safety/pharmacovigilance complaints to be systematically recorded and processed in conformity with regulatory and internal specifications. The aim of this process is to ensure an efficient, secure and legally compliant procedure by means of systematic, traceable measure management.
Every action in the process is automatically documented by the system accordingly. Integrated audit trail functions ensure high traceability.
The digital form is filled out step by step and provided with the relevant entries and data. Fields with a blue background are mandatory. Predefined value sets and drop-down functions make it easy for users to fill out the form.
After processing the individual steps (1. Initiation, 2. Initial assessment, 3. Definition of measures, etc.), the workflow system automatically sends tasks and messages to the responsible agents or workflow participants. Once all steps have been processed, the workflow is considered complete.
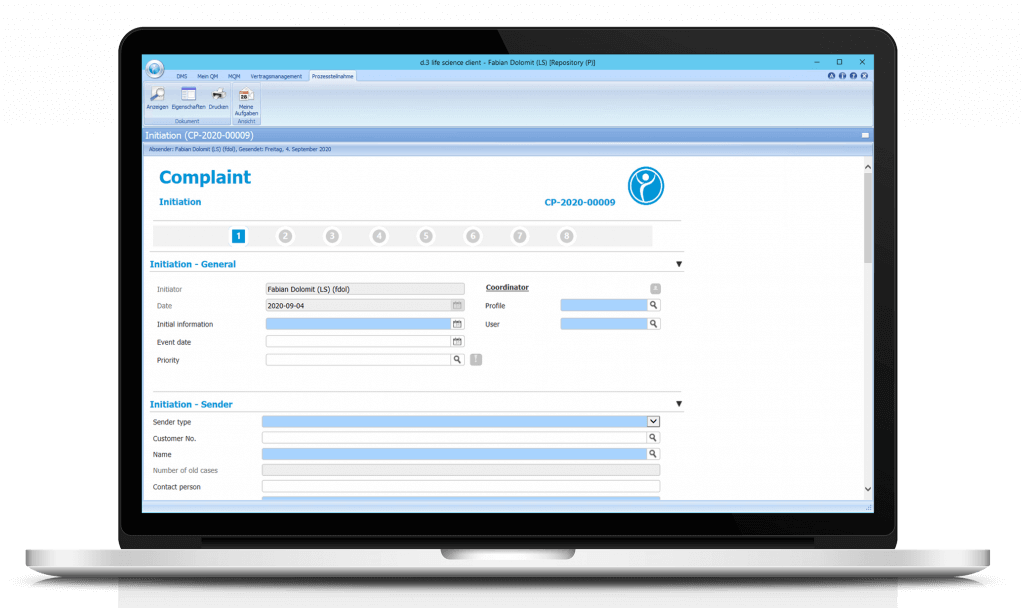
1st Step: Initiation
In this step, the initiator specifies the general data, the sender and the product concerned. If stipulated for the selected product type, the step plan officer will be involved.
Would you like a live insight into the software?
Get a live insight into the possibilities of the production-related QM processes in just 45 minutes with a sample use case. Learn how you can use the solutions of Digital Life Sciences to optimize your production-related QM processes such as complaints (Complaint), deviation reports (Deviation Control), corrective and preventive actions (CAPA) or change controls (Change Control) with our digital workflows.
Some features of Complaint Management
- Definition of affected products/batches and their characteristics
- Evaluation of laboratory results
- Ad-hoc evaluations
- Parallel and serial sending of tasks
- Adding documents, such as digital photos, to your complaint form
- Automatic PDF creation and storage of the form in the eDMS after each step
- Logging of activities in audit trail
- Optional return in process
- Initiation of the individual tasks for the further processing of the workflow
- Integrated absence management
- Distributor for planning, processing and approving measures
- Referencing master data from your ERP system
- Escalation messages both within the system and by e‑mail
What regulations must be observed in the Complaint Process?
- ISO 9001:2015, Chapter 5.1.2
- EU GMP Guide Part 1, Chapter 8
- EU GMP Guide Part 2, Section 15
- FDA 21 CFR Part 211
- FDA 21 CFR 7 Subpart C
- Ordinance for the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients (AMWHV) §19
33 Good reasons for a cooperation with Digital LS
You’re not convinced yet? Find out about 33 good reasons speaking for a cooperation with Digital Life Sciences GmbH. We will show you reasons from the provider’s point of view, from the software point of view and other general reasons that distinguish us.
Customer review
“As an automotive supplier, we really do have very extensive documentation obligations in quality management. In the past, such an amended regulation took a good week to circulate. We have looked at many solutions to optimize processes. But when we saw what the digitalization solutions of Digital Life Sciences can do, we did not even look any further. Everything is now transparently documented, versioned, archived and traceable.”

You might also like…
Complaint is a module of the Digital Life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other types of documents — you can create, revise and sign them all digitally with the document control software.

Training Management software
Extend the “Document Control” module to actively plan and record the qualifications of your employees with our training management software.

E‑learning software
Use the new software “E‑Learning” to train your employees digitally. Create an e‑learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.
Frequently asked questions (FAQs) about Complaint
Can the predefined steps be customized?
The Complaint process flow has been designed in accordance with the GxP regulations and created in consultation with QM experts. Against the background of the validation documentation, the process steps are therefore mandatory. However, each process step contains a ‘Custom Panel’, which can be used to display further information or initiate actions.
Can the predefined designations be changed?
In the workflow administration, you can configure the names of the process steps, field names and datasets.
Are the contents of all process steps visible?
When working on a process step, only this step can be processed, but the other process steps are still visible.
Can I analyze the Complaints?
The essential data of a Complaint is transferred to the attributes of the data record. This allows you to search and analyze the Complaints you have created. The analyses can be exported as PDF, EXCEL or WORD documents.
Can tasks be delegated?
In all process steps that follow the initiation, you can forward the process step to another person. The recipient’s authorization is checked during the selection.
Why does it make sense to use complaint management software?
Product defects affect customer satisfaction and can also pose a risk to health and safety. Complaint management is therefore a central element of quality management: Reported complaints are systematically recorded and processed. A complaint management software partially automates the management process and handles complaints efficiently. It also provides a variety of methods and tools to record incoming complaints, identify their causes, eliminate them and prevent them in the future. This minimizes the risk of repeating errors and reduces the associated costs. The aim is to achieve a sustainable improvement in product quality.
How can the Complaint software support you in your business?
Complaint enables the secure, structured and efficient processing of complaints using electronic signatures and standardized workflows. This creates transparency, documents the decisive processing steps and makes every complaint traceable. In this way, possible irregularities can no longer be overlooked in the future. Early warning indicators and differentiated escalation mechanisms are also proving useful. Responsible parties are notified according to their role so that the complaint process is continuously monitored. In addition, workflows can be flexibly adapted to specific company scenarios. This ensures compliance with applicable industry standards and regulations, such as ISO 9001 or ISO 13485.
Why is the importance of complaints particular in the controlled environment?
In controlled environments, the role of complaints is of particular importance. FDA regulations and ISO standards require a formal complaint management system. Effective complaint management is important for informing supervisors or regulators of quality issues. Controlled companies need to be able to resolve these issues quickly.
Can process steps be subsequently corrected?
In principle, a process step is completed with an electronic signature and can no longer be corrected. With the function ‘Step back’ the process can be returned to a previous process step. The user who has completed this step receives it again for editing and the process continues from this step. Any return to a previous step is recorded in the audit trail.
Can a process be cancelled?
It can be configured so that the process can be cancelled at any process step. For the cancellation, it is required to indicate a reason in a mandatory field. Only the coordinator of the process is allowed to cancel.
Can attachments be stored with a Complaint?
If additional files are required for a process, attachments can be uploaded in all process steps and linked to the current process. The attachments are stored under a corresponding document type in the DMS and can be viewed in the complaint under ‘Attachments’ by all process participants. You can configure which file types can be saved as attachments and how large the files can be.
Are there time limits for the execution of the process steps?
In the administration, time specifications can be entered for each process step. Reminder messages are sent before the process steps are due. The reminder message is sent to the processor of the task, the coordinator and the configured group that is to be informed about the reminder (for example, QA). A reminder is sent with the configured subject.
Are there templates for measures in a Complaint?
Having entered a measure, you can save it as a template in the measure table. The templates are available for insertion in a Complaint. The due date for the measure is calculated based on the duration entered in the template.
Can complaint management (Complaint) be linked to DC, CAPA or Change Control?
In combination with the Deviation Control, CAPA and Change Control workflows, the complaint management software “Complaint” handles the entire problem resolution process, from reporting to effectiveness testing to the complaint completion. For example, appropriate corrective or preventive actions can be created directly from identified deficiencies. The Complaint software thus significantly increases the efficiency of quality-related processes and contributes to a significant reduction in the costs of quality incidents.
To what extent can your complaint management process be optimized by using the Complaint solution?
Within complaint processing, an initial assessment of the situation or case/problem can be mapped by the company’s own employees. In the further course, measures can be defined which consequently contribute to problem solving and thus to complaint stimulation after they have been processed. These are important steps to locate responsibilities and decisions and to avoid errors in the process chain. Ultimately, the ability to ask questions digitally within the process and receive feedback in a short time also proves to be a great opportunity for companies.
Quick contact
Do you have a question about the Complaint module?
Our sales team will help you promptly and gladly.
