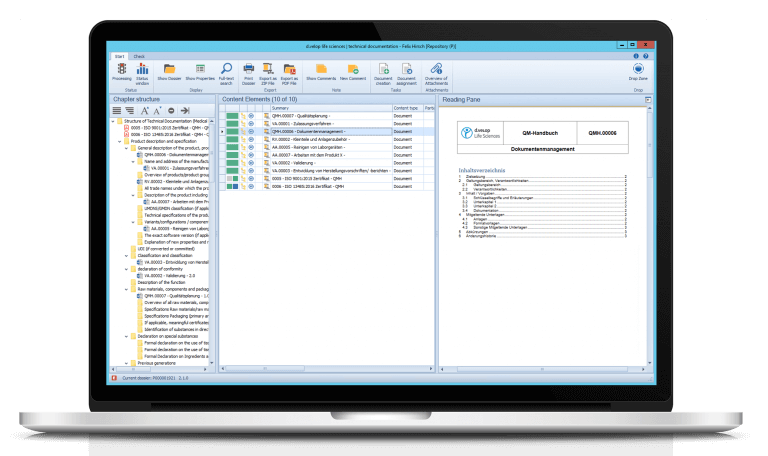
Digital GxP-compliant technical documentation for medicinal products
“Foremost, the flexibility spoke for the d.velop solution. From the very beginning, we could have the document management system customized to our expectations, making it 100 percent suitable for our purposes. Other vendors could not deliver this in the same way.”

Amann Girrbach
The German-Austrian enterprise Amann Girrbach sells medical products globally. The cultures may be different but all nations have one thing in common: Medicinal products must be flawless. Thus, Amann Girrbach does not only have to ensure the excellent quality of its products and services but the enterprise also has to comply with the justifiably high requirements of the respective regulatory authorities. And these requirements have grown for years.
With more than 430 employees worldwide, Amann Girrbach manufactures 10 product groups and therefore has to document hundreds of work steps, recipes, mixture ratios, procedures and measures for the quality assurance. And this for various authorities in different countries. The technical documentation must meet the guidelines for good practice known under the keyword “GxP”.
In Germany, for example, the TÜV Süd is validating medicinal products as well as the Dekra or the TÜV Nord. In the USA, the Food and Drug Administration (FDA) supervises the quality of food and medicinal products with comparable authorities in other countries. Different country, different forms, different requirements — and a high effort for Amann Girrbach to be able to offer various medicinal products worldwide. Especially, as the technical documentation has been conducted on paper in the past.
Keeping the technical documentation for medicinal products up-to-date
“We are working on two premises in Koblach, Austria and in Pforzheim [Germany, the editor]”, Philipp Wildgrube, Regulatory Affairs Manager at Amann Girrbach states. “Over time, it became more and more challenging to keep the technical documentation up-to-date.” Moreover: “Using regular mail, documents took a long time to get from one site to the next.”
Furthermore, Amann Girrbach looked for partner for the digital implementation of their technical documentation. The system had to be flexible and customizable by the user. Additionally, the software vendor should have extensive know-how in the GxP sector.
On the trade fair Control in Stuttgart, Amann Girrbach became aware of the document management system d.velop documents (formerly d.3ecm) of d.velop AG. It allows enterprises to quickly and effectively digitize their information and processes. The document management system can become a central storage location for all information and documents in the company while the industry-specific and GxP-compliant additional modules and services of the Digital Life Sciences successfully adjust it to the individual requirements of Amann Girrbach.
GxP-compliant documentation solution
Digital Life Sciences GmbH is the competence center of d.velop AG for GxP-compliant document solutions and for electronic quality management solutions. With about 60 employees, the Digital Life Sciences supports more than 130 customers, the majority of which is working in the validated GxP sector, e.g. in medical technology or the pharmaceutical industry.
Especially companies in the medical field have to think more and more about the technical documentation of their products. For years now, the regulatory requirements of legislators and testing authorities have been growing here. Here, the document management system helps to keep on top of things. Amann Girrbach benefited from this.
Flexible ECM solution for the pharmaceutical industry
“Foremost, the flexibility spoke for the d.velop solution”, Philipp Wildgrube illustrates. “From the very beginning, we could have the document management system customized to our expectations, making it 100 percent suitable for our purposes. Other vendors could not deliver this in the same way.” The requirements included being able to implement the structure of Amann Girrbach’s technical documentation.
In 2015, Amann Girrbach and the Digital Life Sciences started the common project and began to install the document management system, optimizing processes and digitizing information. Initial successes quickly became apparent, and with the legal framework constantly changing, there is always work to adapt. One of the driving forces behind this was the new European Medical Devices Regulation (MDR) of the year 2017.
Technical documentation 2.0
The requirements that regulatory authorities place on medical companies are varied and in some cases considerable. Among them are original document that must not be changed any more after they have been released. “Our software can implement this,” says Frank Laumann, Head of Service and authorized representative at Digital Life Sciences. The Digital Life Sciences client now allows Amann Girrbach to securely implement their GxP processes.
The benefits of the software include:
- Comprehensive digital management of the dossier
- Digital creation and update of the technical documentation within the requirements of the Medical Device Regulation
- Making cluttered file servers with redundantly stored copies and folder structures a matter of the past
- Minimizing process runtimes for the creation, revision and release of dossiers
- Versioning of the dossier
- Export of the entire dossier
- Checking the dossier for completeness
“The information is now sensibly and logically structured,” Philipp Wildgrube states, “that was nice and easy with and from d.velop. The software allows us to implement almost anything you like.” One of the greatest advantages of the GxP-compliant digital document management system lies in the fast access times. No employee is forced to browse through piles of paper any more or search in the mailboxes. Laumann: “Information can be found quicker, all employees needing access have one central point of entry.”
Support team and contact persons
Fast access to information is one of Amann Girrbach’s most significant requirements with a GxP-compliant document management system. “The big advantage of the d.velop products is that we can quickly and easily access documents.”, Wildgrube describes. He continues: “We are fully satisfied with the working procedures of the system and with the support team.”
Project partners
Since 2007, Digital Life Sciences GmbH is the Competence Center of d.velop AG for GxP-compliant documentation solutions. Thilo Gukelberger, one of the co-founders of the d.velop AG, manages the company along with other long-term employees of d.velop AG. Thus, we are significant co-inventors of the basic product d.velop documents (formerly d.3ecm) which is meanwhile used as a central ECM/DMS archive system by more than 12,500 users globally. The solutions by the Digital Life Sciences GmbH are to be understood as complementary additions to d.velop documents (formerly d.3ecm). They primarily address document-based processes of manufacturing/production and the quality management. In addition to our customers of the classical Life Sciences industry (pharmaceutical, medical technology, food supplements, cosmetics), also customers from the steel industry and the service sector trust the solutions of the Digital Life Sciences GmbH.
Learn more about technical documentation
Get a holistic overview of our MDR-compliant solution now.