Definition of the term (“What is FDA 21 CFR Part 11?”)
FDA 21 CFR Part 11 is a set of regulations introduced by the US Food and Drug Administration (FDA) in 1997. It defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable and equivalent to paper records and handwritten signatures. Essentially, it sets the rules for the use of electronic systems in industries regulated by the FDA.
The introduction of Part 11 was aimed at modernising and streamlining record-keeping processes in the pharmaceutical and life sciences sectors. It should utilize the technology, but at the same time ensure data security and integrity. The necessity for such regulations arose when these industries increasingly introduced electronic methods for recording.
What are the main requirements of FDA 21 CFR Part 11?
- Electronic signatures: One of the central components of FDA 21 CFR Part 11 is the use of electronic signatures. The requirements for the use of electronic signatures are defined in order to be equivalent to handwritten signatures. This includes ensuring the security and authenticity of these signatures.
- Audit trails: FDA 21 CFR Part 11 requires the creation of audit trails for electronic records. These audit trails serve as a chronological record of system activities, enabling traceability and accountability.
- Validation of electronic systems: Companies must validate their electronic systems to ensure that they meet the requirements of Part 11. This includes extensive testing and documentation to prove the reliability and accuracy of the system.
- Access controls: FDA 21 CFR Part 11 also emphasizes access controls to ensure that only authorized individuals have access to electronic records and systems. This improves data security and prevents unauthorized changes.
Your path to digitization — Discover our software
Our digitization solutions primarily address document-based processes in manufacturing, production and quality management. The basis of the dls | eQMS is a holistic ECM/DMS system. The ECM/DMS system can be connected to your existing ERP system (e.g. SAP) and thus map almost all document-based processes in the company.
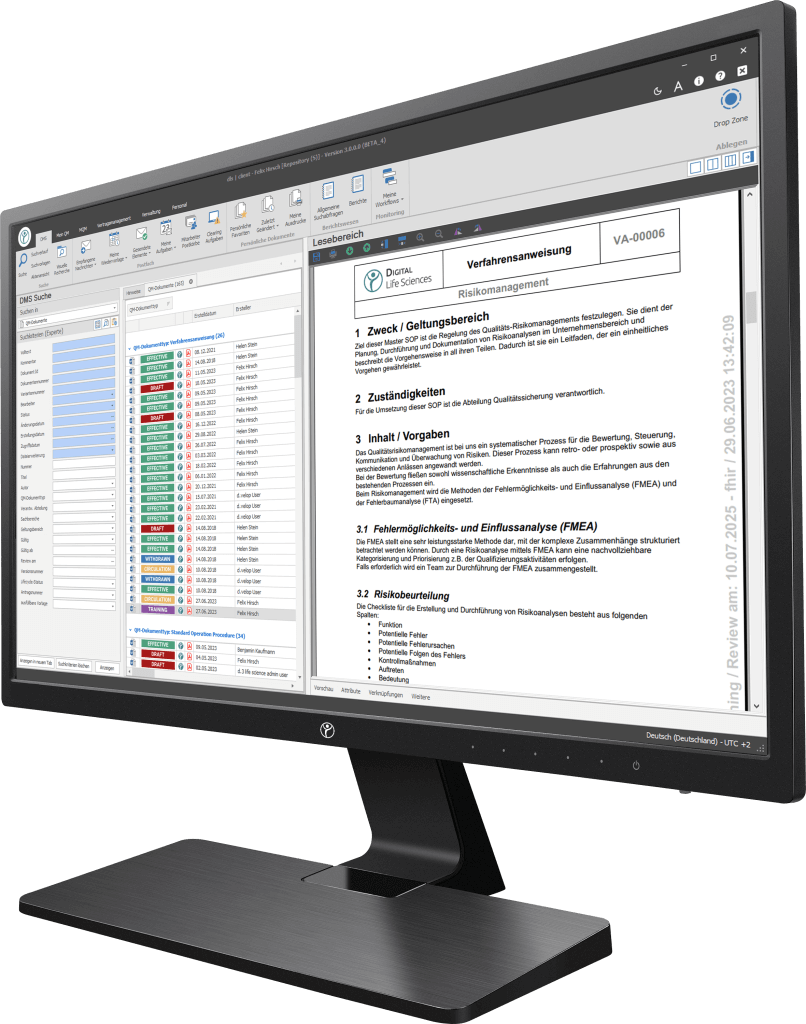
Achieve compliance
Steps to achieve compliance with FDA 21 CFR Part 11:
- Evaluation: Start by evaluating your current systems and processes to identify compliance gaps.
- Validation: Ensure that your electronic systems are validated and that all software is properly tested and documented.
- Access control: Implement strict access controls to protect electronic records.
- Training: Train your staff on Part 11 requirements and best practices for electronic record keeping.
- Audit trails: Establish comprehensive audit trails for electronic records.
- Documentation: Maintain detailed documentation of all processes and system validations.
Advantages of compliance
Compliance with FDA 21 CFR Part 11 holds numerous advantages:
- Improved data security
- Increased data security
- Simplified recording management
- Lower risk of regulatory violations
- Increase in operating efficiency
Conclusion
In a digital age where data is king, FDA 21 CFR Part 11 plays a critical role in ensuring the integrity and security of electronic records in the pharmaceutical and life sciences industries. By complying with their requirements, organizations can not only meet regulatory standards, but also strengthen their data management practices, ultimately leading to more secure and efficient operations.
Start your digital transformation with our powerful, modular software solutions
Frequently Asked Questions (FAQs)
To which industries does FDA 21 CFR Part 11 apply?
FDA 21 CFR Part 11 applies primarily to the pharmaceutical, biotechnology and medical device industries.
Is compliance with Part 11 mandatory?
Yes, compliance with FDA 21 CFR Part 11 is mandatory for organizations operating within its scope.
What are the consequences of non-compliance with FDA 21 CFR Part 11?
Non-compliance may result in regulatory measures, including fines and product recalls.
Are there specific software tools developed for Part 11 compliance?
Yes, there are software solutions aimed at helping companies achieve and maintain Part 11 compliance. You can find some of these tools here.
How often should audit trails be checked?
Audit trails should be reviewed regularly, with the frequency determined by the organization’s risk evaluation and compliance policies.
